MRSA Aggregate in Nooks Inside the Lung
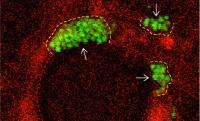
New images reveal how MRSA bacteria (green) aggregate in crevices of the lung and how the aggregates cause damage. Images: Jahar Bhattacharya Lung Biology Lab / Columbia University Irving Medical Center
Using real-time imaging of the inside of the lung, researchers at Vagelos College of Physicians and Surgeons have learned how a dangerous strain of Staph evades the lung’s initial defense mechanisms and within 10 minutes starts a process that severely damages the lung.
Staphylococcus aureus, especially the epidemic USA300 strain, is a major cause of pneumonia in hospital patients and often causes pneumonia in previously healthy people with the flu.
To learn how USA300 first establishes itself in the lung, a team of researchers from the Departments of Medicine and Pediatrics—Jaime Hook, MD, Naeem Islam, PhD, Dane Parker, PhD, Alice Prince, MD, Sunita Bhattacharya, MD, and Jahar Bhattacharya, MD, DPhil—injected fluorescent USA300 bacteria into a live mouse lung. They then watched what happened to the bacteria and the air sacs.
Live imaging of the lung’s intact air sacs is not easy. The Bhattacharya lab has pioneered an approach in which fluorescent dyes can be injected into the air sacs through fine glass needles, making the air sacs optically vivid by confocal microscopy.
The images showed that within 10 minutes of entering the lung, USA300 bacteria bunch up in nooks of the lung’s air sacs. In these aggregates, the bacteria were able to evade one of the lung’s defense mechanisms: a thin layer of flowing liquid that normally sweeps bacteria out of the air sacs.
The USA300 aggregates were also unaffected by the antibiotic vancomycin, which can kill USA300 bacteria in vitro but tends to be ineffective in pneumonia patients.
With their imaging system, the researchers saw that vancomycin fails to fight USA300 in the lung because it cannot penetrate the bacterial aggregates.
Vancomycin only worked against USA300 bacteria in the lung when the bacteria’s PhnD gene had been inactivated or when an antibody that blocked PhnD was introduced into the lung. In vitro studies have shown that PhnD helps link bacteria together into a biofilm; the new study is the first to show that PhnD also works this way inside intact air sacs of the lung.
In people, PhnD is also likely to be critical in establishing USA300 infections: the researchers found that the bacteria also aggregated in nooks inside human lungs.
“The study shows that biofilm-like aggregates are important in establishing USA300 infections in the lung,” says Sunita Bhattacharya, “and that targeting PhnD or finding other ways to break up bacterial aggregates may help prevent USA300-induced lung injury.
References
The study, titled Disruption of staphylococcal aggregations protects against lethal lung injury, was published in the March issue of the Journal of Clinical Investigation.
Jaime Hook is an assistant professor of medicine; Sunita Bhattacharya is an associate professor of pediatrics; Jahar Bhattacharya is a professor of medicine and physiology & cellular biophysics; and Naeem Islam is an associate research scientist in the Department of Medicine, all at the Vagelos College of Physicians and Surgeons. Dane Parker, assistant professor of pediatrics, is now at Rutgers University Medical School.
This work was supported by an American Heart Association Fellow-to-Faculty Transition Award, a Stony Wold-Herbert Fund Inc. Fellowship, and the National Institutes of Health (T32HL105323, R01HL36024, and R01HL122730).
The authors report no conflicts of interest.